9th April 2024
British National Formulary
This update contains 6 significant changes, 5 new monographs, 3…


Psychotropic Drug Directory supports the optimal and rational use of medicines, to improve the quality of life for people with mental health needs.
Close
Close

Contact our team to see how our services can directly help you.
Close

Learn how treatment guidance from Palliative Care Formulary can support health professionals to manage cancer-related neuropathic pain.
Close

The latest new drugs and significant changes to medicines information on MedicinesComplete - monthly.
Close

Read why excipients expert Chris Moreton has enjoyed contributing for the last 30 years.
Close
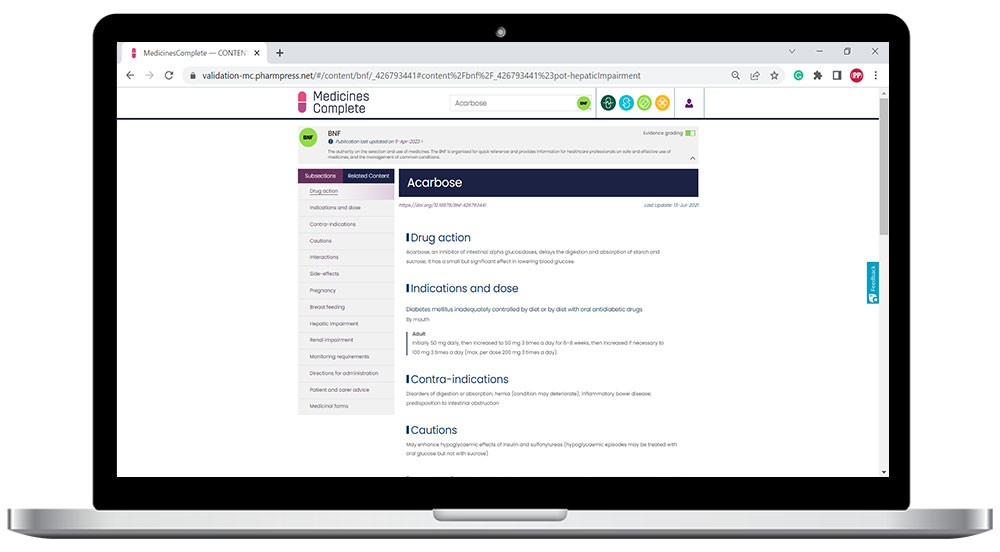

Practical and evidence based, British National Formulary (BNF) is the only drug formulary in the world that is both independent, and has rigorous, accredited content creation processes.
An integral part of the UK’s healthcare infrastructure and relied on by health professionals who prescribe, dispense, and administer medicines globally. Reflecting current best practice as well as legal and professional guidelines relating to the uses of medicines, BNF supports safe and effective decision-making at the point of care.




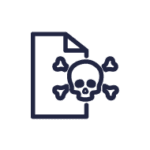


Our knowledge products are regularly updated online through MedicinesComplete
9th April 2024
This update contains 6 significant changes, 5 new monographs, 3…
9th April 2024
This update contains 6 significant changes, 5 new monographs, 3 new preparations, 1 deleted monograph and 2 deleted preparations.
Significant Changes:
New Monographs:
New Preparations: Emylif® orodispersible film [riluzole]; Etacortilen® eye drops [dexamethasone]; Mounjaro KwikPen® [tirzepatide].
Deleted Monographs: Benzoin tincture, compound.
Deleted Preparations: Hibitane® Plus 5% concentrate solution [chlorhexidine]; Zofran® suppository [ondansetron].
12th March 2024
This update contains 5 significant changes, 1 dose change, 4…
12th March 2024
This update contains 5 significant changes, 1 dose change, 4 new monographs and 2 deleted monographs.
Significant Changes:
Aripiprazole (Abilify® and generic brands): risk of pathological gambling [MHRA/CHM advice].
Empagliflozin: update to advice on use in renal impairment, including new indication for chronic kidney disease.
Quinolones: must now only be prescribed when other commonly recommended antibiotics are inappropriate [MHRA/CHM advice] (see example in ciprofloxacin).
Type 2 diabetes: updated guidance for management.
Vitamin B12: advise patients with known cobalt allergy to be vigilant for sensitivity reactions [MHRA/CHM advice] (advice in cyanocobalamin, hydroxocobalamin; see example in hydroxocobalamin).
Dose Changes:
New Monographs:
Deleted Monographs: Cinchocaine hydrochloride with fluocortolone caproate and fluocortolone pivalate; Simvastatin with fenofibrate.
13th February 2024
This update contains 7 significant changes, 5 new monographs, 2…
13th February 2024
This update contains 7 significant changes, 5 new monographs, 2 new preparations, and 3 deleted monographs.
Significant Changes:
New Monographs:
New Preparations: Octasa® MR 1600 mg tablets [mesalazine]; Octasa® suppositories [mesalazine].
Deleted Monographs: Diflucortolone valerate; Fluocinolone acetonide with clioquinol; Fluocinolone acetonide with neomycin.
9th January 2024
This update contains 6 significant changes, 1 dose change, 4…
9th January 2024
This update contains 6 significant changes, 1 dose change, 4 new monographs and 1 new preparation.
Significant Changes:
Dose Changes:
New Monographs:
New Preparations: Zeyzelf® [rivastigmine].
5th December 2023
This update contains 9 significant changes, 2 dose changes and…
5th December 2023
This update contains 9 significant changes, 2 dose changes and 1 new monograph.
Significant Changes:
Dose Changes:
New Monographs:
14th November 2023
This update contains 9 significant changes, 2 dose changes, 4…
14th November 2023
This update contains 9 significant changes, 2 dose changes, 4 new monographs, 4 new preparations and 1 deleted monograph.
Significant Changes:
Dose Changes:
New Monographs:
New Preparations:
Demovo® [desmopressin]; Desmopressin sublingual tablets; Opfolda® [miglustat]; Wegovy® [semaglutide].
Deleted Monographs:
Nandrolone.
Access British National Formulary through MedicinesComplete today.
Contact us now for pricing and access information.
![]()
![]()
BNF content is copyright © BMJ Publishing Group Ltd and Royal Pharmaceutical Society of Great Britain. BNFC content is copyright © BMJ Publishing Group Ltd, Royal Pharmaceutical Society of Great Britain, RCPCH Publications. All rights reserved.
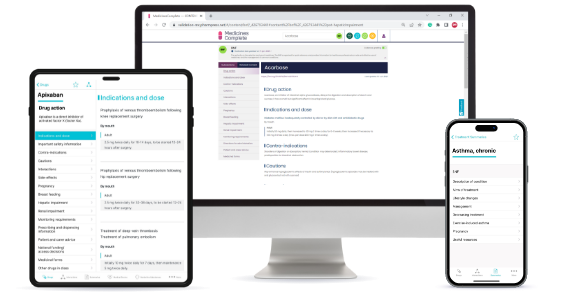
A significant investment from National Institute for Health and Care Excellence (NICE) ensures that, with our partners BMJ Group and Royal College of Paediatrics and Child Health, we support NHS health professionals in the UK with unlimited access to BNF content online through MedicinesComplete, via the BNF + BNFC app.

$99.00
Info
$79.00
Info
$110.00
InfoSee all our printed publications in the Shop.